drawing the outer electron box diagram of a transition metal Transition elements or transition metals. These are metallic elements in which the last electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and ( n – 1) . Joinfworld 6x6x4 Outdoor Electrical Box Weatherproof Junction Box Plastic Clear Waterproof Box Enclosure Nema 4 for Electronics Project WiFi Timer Conduit Marine Outside
0 · transition metal cations diagram
1 · orbital electron structure diagram
2 · orbital electron configuration diagram
3 · orbital diagram of transition metals
4 · orbital box notation of electrons
5 · electron configuration in orbital box
6 · electron configuration diagram pdf
7 · 40 electron configuration diagram
Find WEATHER GUARD Steel truck tool boxes at Lowe's today. Shop truck tool boxes and a variety of automotive products online at Lowes.com.
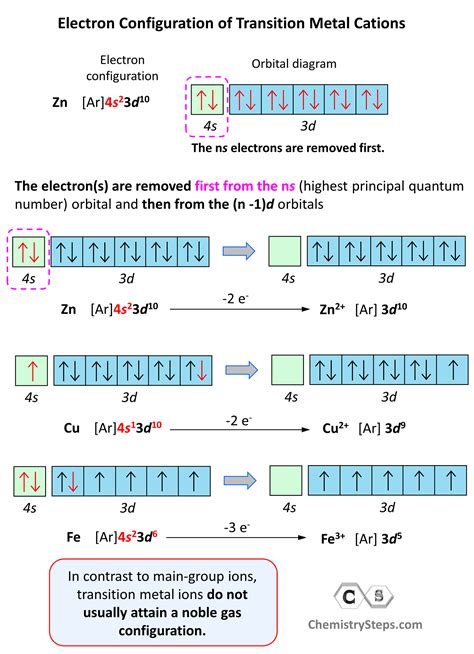
The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing .Question: Drawing the outer electron box diagram of a transition metal cation Draw an outer electron box diagram for a Zrcation. Show transcribed image text There are 2 steps to solve this one. Writing an electron configuration for a transition metal ion starts with the same steps as writing the configuration of an s- or p-block cation: Write the electron configuration for the neutral atom and then determine the number .Our expert help has broken down your problem into an easy-to-learn solution you can count on. Question: Drawing the outer electron box diagram of a transition metal c Draw an outer electron box diagram for a Mn3 cation. Here’s the best .
Transition elements or transition metals. These are metallic elements in which the last electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and ( n – 1) .
Draw an outer electron box diagram for a Tc cation. A + 3 cation of a certain transition metal has two electrons in its outermost d subshell. Which transition metal could this be?
Electron configurations and orbital box diagrams can be written right from the periodic table. The periodic table below, shows the s, p, d, and f-blocks. When reading the periodic table from left to right, one can easily write an electron .Study with Quizlet and memorize flashcards containing terms like Define a transition element, Draw the electron box diagram for chromium and copper, Why is scandium not a transition element? and more.
The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals.Electron Configuration of d Elements – the Transition Series. There is one exception to keep in mind for the electron configuration of transition metals. That is the (n +1)s orbitals always fill before the nd orbitals.Question: Drawing the outer electron box diagram of a transition metal cation Draw an outer electron box diagram for a Zrcation. Show transcribed image text There are 2 steps to solve this one.
The periodic table can be divided into three categories based on the orbital in which the last electron to be added is placed: main group elements (s and p orbitals), transition elements (d orbitals), and inner transition elements (f orbitals). Writing an electron configuration for a transition metal ion starts with the same steps as writing the configuration of an s- or p-block cation: Write the electron configuration for the neutral atom and then determine the number of electrons that are lost to form the cation.Our expert help has broken down your problem into an easy-to-learn solution you can count on. Question: Drawing the outer electron box diagram of a transition metal c Draw an outer electron box diagram for a Mn3 cation. Here’s the best way to solve it.Transition elements or transition metals. These are metallic elements in which the last electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and ( n – 1) d electrons.
Draw an outer electron box diagram for a Tc cation. A + 3 cation of a certain transition metal has two electrons in its outermost d subshell. Which transition metal could this be?Electron configurations and orbital box diagrams can be written right from the periodic table. The periodic table below, shows the s, p, d, and f-blocks. When reading the periodic table from left to right, one can easily write an electron configuration without memorizing the filling order.
Study with Quizlet and memorize flashcards containing terms like Define a transition element, Draw the electron box diagram for chromium and copper, Why is scandium not a transition element? and more.
The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals.
Electron Configuration of d Elements – the Transition Series. There is one exception to keep in mind for the electron configuration of transition metals. That is the (n +1)s orbitals always fill before the nd orbitals.Question: Drawing the outer electron box diagram of a transition metal cation Draw an outer electron box diagram for a Zrcation. Show transcribed image text There are 2 steps to solve this one.
how to cover electrical panel box
The periodic table can be divided into three categories based on the orbital in which the last electron to be added is placed: main group elements (s and p orbitals), transition elements (d orbitals), and inner transition elements (f orbitals). Writing an electron configuration for a transition metal ion starts with the same steps as writing the configuration of an s- or p-block cation: Write the electron configuration for the neutral atom and then determine the number of electrons that are lost to form the cation.
Our expert help has broken down your problem into an easy-to-learn solution you can count on. Question: Drawing the outer electron box diagram of a transition metal c Draw an outer electron box diagram for a Mn3 cation. Here’s the best way to solve it.Transition elements or transition metals. These are metallic elements in which the last electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and ( n – 1) d electrons.Draw an outer electron box diagram for a Tc cation. A + 3 cation of a certain transition metal has two electrons in its outermost d subshell. Which transition metal could this be?Electron configurations and orbital box diagrams can be written right from the periodic table. The periodic table below, shows the s, p, d, and f-blocks. When reading the periodic table from left to right, one can easily write an electron configuration without memorizing the filling order.
transition metal cations diagram
how to connect flexible plastic conduit to electrical box
how to cut a junction box hole in plywood
how to connect two metal electrical boxes
how to cover electrical box with beadboard panel
A weatherproof box is a solution to achieving a weatherproof connection solution. Weatherproof electrical boxes, when appropriately connected, seal out the weather and prevent moisture from getting in. Most weatherproof boxes have a rubber seal to prevent moisture or dust from entering.
drawing the outer electron box diagram of a transition metal|electron configuration in orbital box